Discount Products: Look through products available for a 25% – 50% discount in 2020. The items provided on a first come, first serve basis. View Discounted Products
Reichert SPR – A Vital Tool in Developing Therapeutics and Designing Vaccines
1 –Technical note from Reichert Technologies, 3362 Walden Ave., Suite 100, Depew, NY 14043
INTRODUCTION
Surface Plasmon Resonance (SPR) is an invaluable technique that generates information-rich data for a variety of biomolecular interactions. Researchers utilize SPR to understand biological pathways and to develop and characterize a range of potential therapeutics to treat disease and other illnesses. In the context of the current coronavirus global pandemic, SPR can be employed to develop potential therapeutics to treat COVID-19 disease and help engineer vaccines to prevent virus infections. It can reveal vital structural and function information between the pathogen and host immune system that is critical in therapeutic development. SPR can be used to explore a variety of potential therapeutics that include low molecular weight compounds or drugs, peptides, proteins, and antibodies.
REICHERT SPR IN MALARIA VACCINE DEVLOPMENT
The importance of using SPR in guiding vaccine development is highlighted in two recent articles where researchers used a Reichert SPR system to investigate Malaria treatment.1,2 An integral step in malaria vaccine development has been to understand how infection by parasites occurs. Of the known human malaria parasites, the group whose research is highlighted here has targeted two – Plasmodium falciparum (P. falciparum)1, which is the most deadly parasite, and Plasmodium vivax (P. vivax)2 which is the most common parasite species that infects humans. A key question that needed to be answered early on is – How do parasites gain entry into red blood cells? This group discovered that P. vivax enters young red blood cells by making proteins that recognize and bind to receptors on the young red blood cell surface. This family of proteins was structurally similar to those used by P. falciparum to infect red blood cells. The next step in their research effort was to determine the three-dimensional structure of the proteins. This determination showed that the proteins are folded in the same way with the main difference being in the electrical charge on the surface of the molecules.
Researchers found that P. falciparum reticulocyte binding protein-like homologue 4 (PfRh4) binds to complement receptor 1 (CR1 or CD35) to mediate entry of malaria parasites into human red blood cells1. CR1 (complement receptor 1) is composed of 28–30 structural modules (which are called complement control protein (CCP) domains) in the extracellular domain. The CR1 protein fragments in the study outlined here consisted of only two CCP domains and had molecular weights of around 14,000 Daltons. Initial mapping studies identified the first two to three N-terminal modules of CR1 (CCPs 1-2 or CCPs 1–3) as specific inhibitors of the PfRh4-CR1 invasion pathway1. Seven different mutant proteins were tested for their inhibitory potential via SPR. SPR was able to rank them in terms of affinity with three having the highest affinity for the intended target. The SPR results matched with the expected biological activity determined independently with other techniques1. Binding affinities for a series of protein fragments to PfRh4 were determined. An example of responses from one of the higher affinity fragments is shown in Figure 1 (CR1 CCP 1-2). Data is fit to a 1:1 binding model with mass transport model:
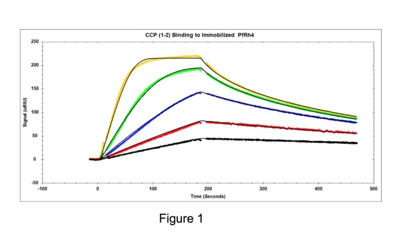
Figure 1: The following concentrations of the CR1 CCP1-2 inhibitor were injected over immobilized PfRh4: 5, 10, 20, 40 and 80 nM. Tracedrawer (Ridgeview Instruments AG) was used to fit the data. The calculated ka is 2.10 e6 M-1 s-1, the kd is 1.26 e-2 s-1, and the equilibrium dissociation constant KD is 6.01 nM.
This information is an important starting point that will lead to further screening of potentially more powerful inhibitors and can aid in the rational development of a novel malaria vaccine.
REICHERT SPR IN HIV/AIDS THERAPEUTIC DESIGN
Treatment with antiretroviral drugs is the accepted approach to HIV/AIDS therapy. Drugs are usually taken in combinations that include nucleoside reverse transcriptase inhibitors, which block how the HIV genetic material is used to create DNA from RNA, protease inhibitors, where the raw protein material for newly produced HIV viral particles is cut into specific pieces, and integrase inhibitors, which prevent the proviral HIV DNA from integrating into the host cell genome. These drugs do not provide a cure but significantly help slow the disease’s progression although with significant side-effects. Researchers in this current study (3) ultimately would like to develop a new class of antiretrovirals that can interfere specifically with the functions of HIV1/Nef. Since Nef is critical for HIV-1 replication in vivo and the immune escape of HIV-infected cells which leads to AIDS progression, development of potent HIV-1/Nef inhibitors hold promise in the suppression of HIV replication and the restoration of immune recognition of HIV-positive cells so that the patient’s own CTL response can be used to combat the disease.3 In this current work, Hydrogen Exchange Mass Spectrometry (HX MS) and SPR have been the primary techniques used to better understand the mechanism of action on Nef conformational transitions in solution by focusing on binding of Src-family kinases. Hence, this study supports the broader possibility that compounds interfering with conformational transitions in HIV-1/Nef structure have potential as a new class of antiretroviral agents.3 This is important as HIV can become resistant to existing treatments over time and new treatments will continue to be needed.
Researchers were interested in knowing whether binding properties changed depending on whether the Nef protein was full-length or truncated so that only the folded core lacking the N-terminal arm responsible for membrane anchoring (amino acids 1-60) was present. They also investigated a mutant defective for homodimer formation, in which Nef Asp123 was changed to Asparagine (Nef-D123N). Substitution of this highly conserved Aspartate prevented dimerization in cell-based fluorescence complementation assays and blocked important Nef functions related to infectivity and replication, as well as CD4 and MHC-I downregulation.3
After immobilization of SH3 or SH3-SH2 domains of Src-family kinases ligand, Nef protein was flowed over the chip either as full- length analyte, wild type core or D123N core. Examples of responses seen for the wild type Nef core are shown below in Figures 2 and 3. When SH3 alone was coupled, the wild type Nef core exhibited single-site binding with a KD value of of 128 nM. Binding was similar for the other Nef proteins – the affinity was a little lower for the full-length Nef (180 nM) and a little higher for the D123N core (119 nM). When SH3-SH2 was employed as the ligand, 2-site binding (conformational change) was seen with an affinity of 475 nM for the Nef core and similar affinities for the other Nef proteins (429 nM and 475 nM for the full-length Nef and D123N core, respectively). Hence, it was found that the mutation had little impact on the kinetics or affinity of SH3 domain interaction, suggesting that the D123N mutation does not influence the conformation of Nef to inhibit binding. This finding was consistent with HX MS results.3
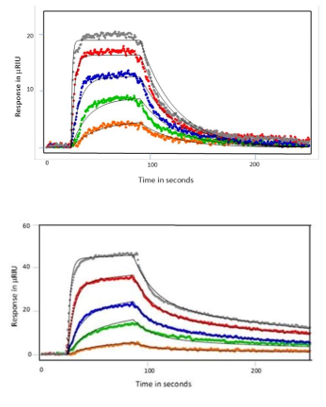 Figure 2: SH3 protein binding to Nef wild type core fit to a 1:1 binding model. Kinetic Rate constants determined in Tracedrawer are as follows: ka = 2.24 x 105 M-1s-1 and kd = 2.76 x 10-2 s-1 with an equilibrium dissociation constant (KD) of 128 nM.
Figure 2: SH3 protein binding to Nef wild type core fit to a 1:1 binding model. Kinetic Rate constants determined in Tracedrawer are as follows: ka = 2.24 x 105 M-1s-1 and kd = 2.76 x 10-2 s-1 with an equilibrium dissociation constant (KD) of 128 nM.
Figure 3: SH3-SH2 proteins binding to Nef wild type core fit to a 2-state conformational change model. Kinetic rate constants determined in Tracedrawer are as follows: ka1 = 6.29×104 M-1s-1, kd1 =5.65×10-2 s-1 and ka2 =1.06×102 M-1s-1, kd2 = 4.72×10-3 s-1 with an equilibrium dissociation constant of KD of 475 nM.
REICHERT SPR IN MONOCLONAL THERAPEUTIC ANTIBODY DEVELOPMENT
Transmembrane 4 superfamily member 5 protein (TM4SF5) is an important possible target for hepatocellular carcinoma and colon cancer. In this work, researchers utilized a cyclic peptide that mimics a structural motif of TM4SF5 as an antigen and then developed a monoclonal antibody that recognizes the TM4SF5 protein.4 The researcher employed Reichert SPR to characterize the antibody and showed that it has a very slow off rate making it an attractive antibody for potential therapeutic outcome. In this study, it was shown that both the cyclic vaccine and the humanized anti-TM4SF5 antibody suppressed the formation and growth of lung metastases that were established through intravenous injection of colon cancer cells in a mouse metastases model.4
CONCLUSION
SPR can play a pivotal role in therapeutics development and vaccine design. Reichert’s SPR systems have been implemented in a variety of research application (see https://www.reichertspr.com/publications/) and have the sensitivity and performance characteristics to meet challenging experimental needs. Reichert offers two main SPR platforms, the 2SPR and 4SPR systems that are affordable, flexible and have outstanding performance. Please contact us to learn more about how Reichert SPR can help you with your therapeutic and vaccine research during this critical time.
REFERENCES
- Nicholas T. Y. Lim, Markus J. Harder, Alexander T. Kennedy, Clara S. Lin, Christopher Weir, Alan F. Cowman, Melissa J. Call, Christoph Q. Schmidt and Wai-Hong Tham, Characterization of Inhibitors and Monoclonal Antibodies That Modulate the Interaction between Plasmodium falciparum Adhesin PfRh4 with Its Erythrocyte Receptor Complement Receptor 1,” J. Biol. Chemistry (2015) Vol. 290, pp. 25307-25321, doi:10.1074/jbc.M115.657171.
- Jakub Gruszczyk, Usheer Kanjee, Li-Jin Chan, Sébastien Menant, Benoit Malleret, Nicholas T. Y. Lim, Christoph Q. Schmidt, Yee-Foong Mok, Kai-Min Lin, Richard D. Pearson,,10 Gabriel Rangel, Brian J. Smith, Melissa J. Call, Michael P. Weekes, Michael D. W. Griffin, James M. Murphy, Jonathan Abraham, Kanlaya Sriprawat, Maria J. Menezes, Marcelo U. Ferreira, Bruce Russell, Laurent Renia, Manoj T. Duraisingh, Wai-Hong Tham1, “Transferrin receptor 1 is a reticulocyte-specific receptor for Plasmodium vivax,” Science (2018), Volume 359, pp. 48-55.
- Jamie A. Moroco, John Jeff Alvarado, Ryan P. Staudt, Haibin Shi, Thomas E. Wales, Thomas E. Smithgall, John R. Engen, “Remodeling of HIV-1 Nef Structure by Src- Family Kinase Binding,” Journal of Molecular Biology, Available online 16 December 2017, ISSN 0022-2836, doi.org/10.1016/j.jmb.2017.12.008.
- Guang Wu, Dongbum Kim, Byoung Kwon Park, Sangkyu Park, Ji-Hee Ha, Te Ha Kim, Avishekh Gautam, Jung Nam Kim, Su In Lee, Han-Bum Park, Yong-Sung Kim, Hyung-Joo Kwon, Younghee Lee, “Anti-metastatic effect of the TM4SF5-specific peptide vaccine and humanized monoclonal antibody on colon cancer in a mouse lung metastasis model,” Oncotarget (2016), Volume 7, pp. 79170 – 79186.
DLS at High Concentration
Dynamic Light Scattering (DLS) is an effective measurement technique used for measuring the hydrodynamic size of common nanomaterials including colloids, nanoparticles, proteins, and polymers. Despite the versatility of this technique, there are several important considerations that cannot be ignored when using light scattering to characterize high-concentration solutions. While it is possible to make measurements on high volume-fraction samples without dilution, it raises additional questions about the meaning of the hydrodynamic size. To understand why this is the case we need to discuss two effects encountered in concentrated solutions: multiple scattering and mutual diffusion.
Download the PDF White Paper to Learn More
Applications of the NanoRack™ Sample Stretching Stage to a Commercial Impact Copolymer
Dalia G. Yablon and Andy H. Tsou, ExxonMobil Research and Engineering, Clinton, NJ
|
A commercial impact copolymer (ICP), amulticomponent material typically used inautomotive and appliance applications where a balance of stiffness and toughness is needed, was studied with the NanoRack™ Sample Stretching Stage accessory on the MFP-3D™Atomic Force Microscope to investigate material deformation and interface adhesion as a function of tensile stress. Effects of deformation were observed within both the polypropylene and ethylene-propylene components, as well as at the interface between the two materials. There are no other direct measurement methods available to determine interfacial adhesive strength of polymer blends, and so AFM investigations of micro-domain deformation such as the one described here could be used ultimately to provide a direct determination of interfacial adhesion in complex polymer containing materials such as ICP. Studies of this kind improve our understanding of material structure-propertyrelationships, ultimately enabling manufacture of better quality products. Application to Impact Copolymer (ICP)The commercial impact copolymer used for this study is composed of a polypropylene (PP) matrix with micron-sized domains of ethylene-propylene (EP) rubber domains produced in a serial polymerization reactor. Dogbone-shaped samples were molded of the impact copolymer measuring at ~20mm (middle straight part of dogbone) by ~4mm in width by 0.2mm thickness. A portion of the straight part of the dogbone was cryo-faced at -120°C with a cryomicrotome to ensure a smooth sample and to remove the thin polymer |
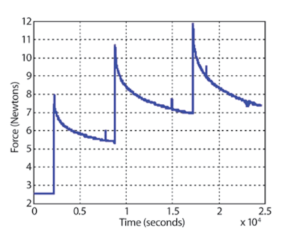
Figure 1: Stress (Newtons) vs. time (seconds) curve of ICP as it is being stretched on the NanoRack. surface layer that forms during the compression molding process (also referred to as a ‘polymer skin’), leaving a small and smooth surface area in the middle of the dogbone that was suitable for imaging. The sample was mounted into a NanoRack Sample Stretching Stage with smooth grips. The NanoRack is a high-strain, high-travel manual stretching stage that provides two-axis stress control of tensile loaded samples and also allows control of the sample image region under different loads. Automatic load cell calibration provides integrated force measurements with MFP-3D images or other measurements and returns both stress and strain data. Figure 1 shows real-time stress vs. time curves of the ICP as the sample is being pulled in the NanoRack. The baseline force is |
AFM Characterization of Thin Films: High-Resolution Topography and Functional Properties
|
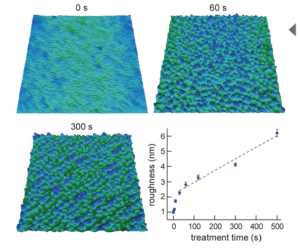
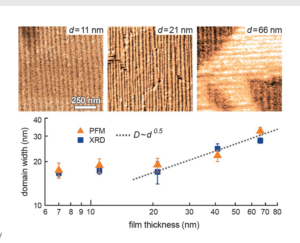
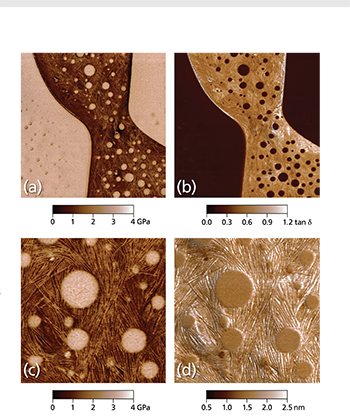
Asylum Research Cypher™ and MFP-3D™ atomic force microscopes (AFMs) provide valuable information for characterizing thin films and coatings. They quantify 3D roughness and texture with unmatched spatial resolution and measure nanoscale functionality including electrical, magnetic, and mechanical behavior. Thin films and coatings play a critical role in everything from food containers to photovoltaics. To meet such varied needs, they are made from every class of material and by numerous processes including physical and chemical vapor deposition techniques, atomic layer deposition, and sol gel processing.1 A key step in developing any new film is characterizing its surface structure and physical properties, whether in engineering commercial products (Figure 1) or pursuing fundamental materials science (Figure 2). The intrinsic dimensions of films (thickness, grain and domain sizes, etc.) make it important to characterize them on sub-nanometer to micrometer length scales. The AFM is a powerful tool for this purpose for many reasons. For instance, it possesses much higher spatial resolution than other stylus or optical-based methods.4 Samples need not be optically reflective or electrically conducting, allowing access to virtually any film. AFMs also provide complementary information to electron microscopes, such as accurate 3D surface profiles, and offer a more flexible operating environment for work at both ambient and non-ambient atmospheres and temperatures. Here we describe the extensive features of Asylum Research AFMs for thin film characterization and show examples over a range of applications. With today’s AFMs, surface roughness can be measured more accurately, quickly, and easily than ever before. A wider array of built-in analysis tools and automated routines mean higher productivity and greater ease of use. Also, research and instrumentation advances have created a variety of AFM modes for measuring nanoscale film functionality including electrical, magnetic, and mechanical response.
|
|
The NanomechPro ™ Toolkit:
Accurate Tools for Measuring Nanoscale Mechanical Properties for Diverse Materials
Understanding nanoscale mechanical properties is of fundamental importance for evaluating the behavior and performance of a wide  variety of industrially, biologically and structurally important materials. An Atomic Force Microscope (AFM) tip interacting with a sample experiences forces originating from many different sources – elasticity, viscosity, adhesion, van der Waals – to name a few. Hence, it has become increasingly clear that reliable and accurate materials properties measurements require looking at your sample in more than one way. Single techniques are simply insufficient for accurately and rigorously revealing sample properties and can often yield misleading and even inaccurate results and conclusions.
variety of industrially, biologically and structurally important materials. An Atomic Force Microscope (AFM) tip interacting with a sample experiences forces originating from many different sources – elasticity, viscosity, adhesion, van der Waals – to name a few. Hence, it has become increasingly clear that reliable and accurate materials properties measurements require looking at your sample in more than one way. Single techniques are simply insufficient for accurately and rigorously revealing sample properties and can often yield misleading and even inaccurate results and conclusions.
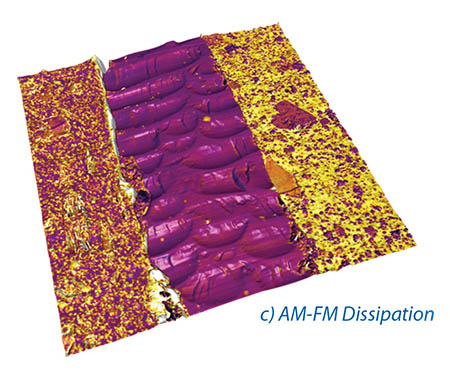
The NanomechPro™ toolkit (Figure 1) for Asylum’s Cypher™ and MFP-3D™ AFMs provides a suite of tools to meet the requirements of the nanomechanics researcher and is both impressively powerful and rapidly expanding. The various tools are complementary – each technique probes and records different responses of your samples – and often can be used imultaneously (e.g. Figures 2a – d). Additionally, with the Cypher AFM, many of these new techniques can be combined with small, fast, low noise cantilevers, enabling measurements at noise levels and speeds previously impossible.
Combined Loss Tangent and AM-FM Imaging
Amplitude-modulated (AM) atomic force microscopy, also known as tapping mode or AC mode, is a proven, reliable and gentle imaging method with widespread applications. Previously, the contrast in tapping mode has been difficult to quantify. However, in this work we introduce two new techniques that allow unambiguous interpretation of material properties in tapping mode: AM-FM and Loss Tangent. Because these measurements are made simultaneously, there is a built-in check for self-consistency in the measurements. The new AM-FM imaging technique combines the features and benefits of normal tapping mode with the quantitative, high sensitivity of Frequency Modulation (FM) mode. Both Loss Tangent and AM-FM imaging can be performed simultaneously at high data acquisition rates. These techniques are exclusively available from Asylum Research, US patents 8,024,963, 7,937,991, 7,603,891, 7,921,466 and 7,958,563 with others pending.
Contact Resonance Viscoelastic Mapping Mode
Introduction
Asylum Research’s Contact Resonance Viscoelastic Mapping Mode option forthe MFP-3D™ and Cypher™ S atomic force microscopes (AFMs) enables high resolution, quantitative imaging of both elastic storage modulus and viscoelastic loss modulus. It is just one of the many anomechanical tools in Asylum’s NanomechPro™ Toolkit. The contact resonance technique is particularly well suited for characterizing moderate to high modulus materials in the range of about 1GPa to 200GPa. Thanks to recent advances by Asylum and our collaborators, Contact Resonance Viscoelastic Mapping Mode is now faster, more quantitative, and easier to use than earlier implementations.
How it works
The contact resonance principle
The contact resonance technique, first developed in the 1990s by the Yamanaka and Arnold groups,1,2 is based on the principle that the resonance of an AFM cantilever changes when it is in contact with a sample. As shown in Figure 1, the cantilever and sample in contact can be thought of as one spring coupled in series to a second spring and dashpot in parallel. Here, the first spring represents the elastic response of the cantilever and the second spring and dashpot represent the viscoelastic response of the sample. Therefore, as the stiffness of the sample contact changes, the frequency of the contact resonance changes (higher stiffness = higher frequency). Changes in the viscous response of the sample are reflected in the Quality factor (Q) of the contact resonance (more viscous = lower Q). Standard contact mechanics models can be used to then convert these stiffness and dissipation measurements to elastic modulus and loss modulus.
Contact resonance imaging
The contact resonance technique is based on contact mode imaging. This means that the AFM cantilever scans along the sample surface at a constant force (the setpoint), measured by the cantilever deflection. As the tip encounters higher (or lower) features, the tip-sample force begins to increase (or decrease). A feedback loop continuously adjusts the height up (or down) to keep the force at a constant value (the setpoint). This motion is recorded as the sample topography.
While the tip scans the sample in contact mode, the contact resonance is continuously changing with the sample mechanical properties. In order to measure the contact resonance, a very low amplitude vertical modulation is introduced by driving either the cantilever or the sample. The drive is at a relatively high frequency, so this modulation does not affect the contact mode feedback loop, but the modulation nevertheless couples to the cantilever deflection and can be measured using a lock-in amplifier.
New Scanning Probe Techniques for Analyzing
Organic Photovoltaic Materials and Devices
Rajiv Giridharagopal, Guozheng Shao, Chris Groves, and David S. Ginger Department of Chemistry, University of Washington, Seattle, WA 98195, USA
Abstract
Organic solar cells hold promise as an economical means of harvesting solar energy due to their ease of production and processing. However, the efficiency of such organic photovoltaic (OPV) devices is currently below that required for widespread adoption. The efficiency of an OPV is inextricably linked to its nanoscale morphology. High-resolution metrology can play a key role in the discovery and optimization of new organic semiconductors in the lab, as well as assist the transition of OPVs from the lab to mass production. We review the instrumental issues associated with the application of scanning probe microscopy techniques such as photoconductive atomic force microscopy and time-resolved electrostatic force microscopy that have been shown to be useful in the study of nanostructured organic solar cells. These techniques offer unique insight into the underlying heterogeneity of OPV devices and provide a nanoscale basis for understanding how morphology directly affects OPV operation. Finally, we discuss opportunities for further improvements in scanning probe microscopy to contribute to OPV development. All measurements and imaging discussed in this application note were performed with an Asylum Research MFP-3D-BIO™ Atomic Force Microscope.
Introduction
OPV materials are an emerging alternative technology for converting sunlight into electricity. OPVs are potentially very inexpensive to process, highly scalable in terms of manufacturing, and compatible with mechanically flexible substrates. In an OPV device, semiconducting polymers or small organic molecules are used to accomplish the functions of collecting solar photons, converting the photons to electrical charges, and transporting the charges to an external circuit as a useable current.1-3
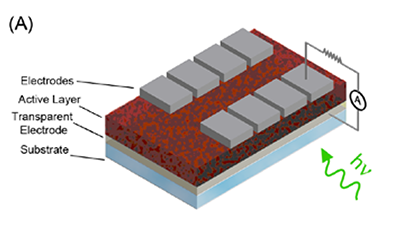
At present, the most intensely-studied and highest-performing OPV systems are those that employ bulk heterojunction (or BHJ) blends as the active layer, with NREL-certified power conversion efficiencies improving seemingly monthly, and currently standing at 6.77%.4 In a bulk heterojunction blend, the donor and acceptor material are typically mixed in solution, and the mixture is then coated on the substrate to form the active layer. The donor/acceptor pair can consist of two different conjugated polymers, but it is often a conjugated polymer (donor) and a soluble fullerene derivative (acceptor).
AM-FM Viscoelastic Mapping Mode
Information on mechanical properties is important in many applications. AM-FM Viscoelastic Mapping Mode lets you quickly and gently image viscoelastic properties including storage modulus and loss tangent with nanoscale spatial resolution. Its very wide operating range, from less than 1 MPa to hundreds of GPa, makes it a highly versatile technique. AM-FM Mode is available on all MFP-3D™ and Cypher™ family AFMs and is one of many options in Asylum’s NanomechPro™ Toolkit for nanomechanical measurements.
Capabilities and Benefits
Asylum’s exclusive AM-FM Viscoelastic Mapping Mode1 is a flexible, convenient tool for nanomechanical characterization. With a range of applicability that spans a remarkable six orders of magnitude in storage modulus (from less than 1 MPa to hundreds of GPa), it is a general-purpose technique for anything from biomaterials and polymers to metals and ceramics. AM-FM Mode provides elastic information including storage modulus, Young’s modulus, and contact stiffness and viscoelastic information including viscoelastic loss tangent and loss modulus. AM-FM Mode gets results by operating at two cantilever resonances simultaneously. As the name indicates, the first resonance is used for tapping mode imaging, also known as amplitude modulation (AM), while a higher resonance mode is operated in frequency modulation (FM). At resonance, the cantilever frequency and phase respond sensitively to changes in sample properties. Small frequency and phase shifts can be measured with very high precision and accuracy, reducing uncertainty and increasing sensitivity. You can use raw output signals to quickly visualize relative contrast and identify sample components; or you can use the observed amplitude, phase, and frequency data to make quantitative estimates of mechanical properties based on built-in or your own models.
Because AM-FM Mode works like tapping mode in the repulsive regime, it is familiar and straightforward to use. It also has the other advantages of tapping mode including fast scanning, high spatial resolution, and gentle forces. On high speed, low-noise systems such as Asylum’s Cypher S and ES AFMs, modulus mapping in AM-FM Mode can routinely operate at line scan rates as fast as 20 Hz (equivalent tip velocity 300 μm/s) and forces as low as 50 pN.2 Low forces mean less sample deformation, typically only a few nanometers, which both minimizes damage and maximizes spatial resolution. Because the FM amplitude is just a tiny fraction of the AM amplitude and is at a different frequency, topographic imaging operates the same as in standard tapping mode. This makes AM-FM Mode very stable and reliable to operate.
Force Scanning with the MFP-3D™ AFMs: Two Capabilities In One
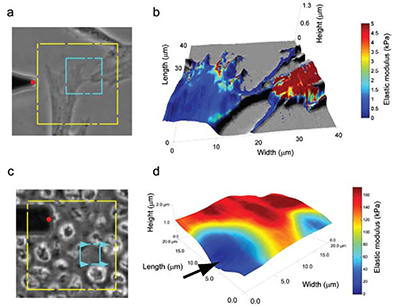
Atomic force microscopy (AFM) is able to reveal many properties about a material. Most commonly, it is used to obtain topographical information, but it can also probe mechanical stiffness, electrical conductance, resistivity, and magnetism. Researchers have used it to study interactions between enzymes and their substrates1, structural changes in injured or diseased tissue2, macromolecular interactions between lipids3 and analysis of nucleic acid organization and structure4, to name a few applications. AFM performs analyses on a micro and nanoscale, allowing it to quantify phenomena as miniscule as van der Waals forces, electrostatic interactions, and molecular bonds5. AFM is also able to produce high-resolution, detailed images of sample surfaces, displaying micro and nanoscale properties of materials as flat as cleaved mica or as non-uniform as a cell. An interesting aspect to AFM is its ability to measure multiple micro- and nanoscale properties in a single test on samples that are unfixed, unstained, and alive. Of particular use in many fields is the imultaneous measurement of topographical features and mechanical properties.
Traditional light microscopy is able to reveal a wealth of information about a sample, especially a biological one. Light microscopy can tell investigators the shape of a cell, localization of subcellular structures within the cell, and even organization of cellular infrastructure, among many other parameters. But a limitation with these optical data is that we are unable to measure, in a directly quantifiable way, the mechanical properties of that cell; these properties give investigators important information about the cell’s cytoskeletal organization and phenotype. The cell’s stiffness, quantified by measuring the elastic modulus of the cell, is different at various points across its surface; cells tend to be softer over the cytoplasm and stiffer over cytoskeletal structures. Generally speaking, AFM is able to assess both mechanical and topographical properties of any material, including cells, simultaneously in a single assay.