Discount Products: Look through products available for a 25% – 50% discount in 2020. The items provided on a first come, first serve basis. View Discounted Products
Apium M220 Series
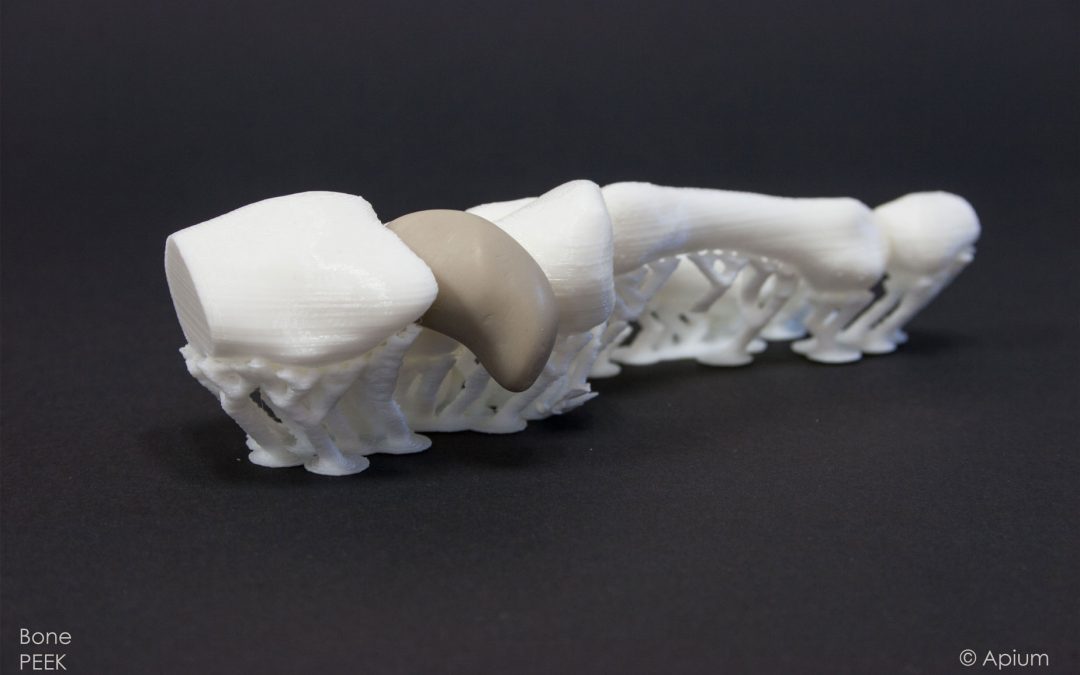
Medical PEEK 3D Printing
Apium is proud to introduce Apium M220 Series 3D Printers – the first of its kind.
Apium M220 is able to process medical grade PEEK, without affecting its biocompatibility and sterilisation properties.
Introducing the Apium M220 Series
Apium hits medical-grade milestone for 3D printed PEEK filament
German 3D printer and high performance materials manufacturer Apium Additive Technologies has hit a new milestone in the processing of a PEEK material filament for medical applications. The company’s Medical PEEK 3D Printing, on the Apium M220 Series machine, has met biocompatibility criteria needed to make implants and devices used in the skull and the hand. Using a filament for FFF desktop 3D printing, this development from Apium promises to “revolutionize patient-specific PEEK implant and medical device production” facilitating professional access to high performance, medical grade materials. Findings of the most recent biocompatibility tests, implants and devices made with medical grade PEEK filament using the Apium M220 3D printer will be shown next week at Formnext 2018 in Frankfurt.


Key Features
Temperature Management System
Apium M220 Series 3D printers are equipped with advanced temperature management system enabling the temperature control of the printed medical device. The atmosphere around the printed medical devices is filtered to prevent contamination.
Advanced Mechanical Parts
Years of experience in materials science and German engineering is combined for the development of mechanical parts on the Apium M220 Series. Sterilisable medical grade materials have been used for the development of the nozzle and print-bed technology.
Certifications
Apium is an ISO9001:2015 certified company. Apium M220 Series 3D printers do not fall under the Medical Devices Directive, but it is suitable for cleanrooms and the production of medical devices. The necessary certificates of biocompatibility for the material and device are available with the product delivery
Key Benefits
Life Saving Innovation
We offer the unique possibility to produce patient specific prostheses and implants at low costs and minimal lead times, without affecting PEEK`s biocompatibility and sterilisation properties. Therefore, the part meets the high requirements for human in vivo applications.
Based on the design freedom that comes along with our technology, complex geometries can be created on your desktop using radiological imaging data as input. The digital implant model, generated from the patient-specific data, can be manufactured from PEEK within a few hours, sterilized then deployed for patient treatment.
The opportunities which Apium’s Medical PEEK 3D Printing Technology offers, pertains most to the craniomaxillofacial, hand and spine areas where patient specific solutions are in most demand. By implementing the Apium solution, accruable savings on time, manufacturing cost and personnel can be realized relative to traditional manufacturing solutions. The Apium solution makes possible a faster and more flexible reaction scenario at times of emergency.
Trust the Experts at Spectra Research Corporation
Spectra Research Corporation (SRC) offers a range of innovative high-quality scientific products and laboratory services to industrial and scientific markets throughout Canada.
Specifications
Apium has developed M220 Series 3D printers for researching, developing and producing PEEK medical devices. We will supply natural colored, high viscosity polyether ether ketone (PEEK) Filament. This filament is specially designed for long term implantable medical devices.





