Additive manufacturing (AM), also known as 3D printing, is a transformative/disruptive approach to industrial production in a broad range of fields that cost-effectively enables the creation of lighter, stronger, and more geometrically complex parts and systems. It is another technological advance made possible by the digitization of processes. AM uses data computer-aided-design (CAD) software or 3D object scanners to direct hardware to deposit material, layer upon layer, in precise geometric shapes. As its name implies, AM adds material to create an object. By contrast, when you create an object by traditional analog methods, it is often necessary to remove material through milling, machining, carving, shaping or other means.
The ability to design and print virtually any object shape using a diverse array of materials, such as metals, polymers, bioinks—and ceramics—has given rise to the use of AM in biomedicine in both research and clinical settings. The world of 3D ceramic printing has come a long way since the 1980s, when it was considered suitable only for the production of functional or aesthetic prototypes, and a more appropriate term for it at the time was “rapid prototyping.” This article reviews the additive manufacturing of ceramics in biomedicine, as well as the technology and products of a leading SRC supplier, 3DCeram.
The additive manufacturing of ceramics for biomedical applications allows for the creation of bone substitutes, custom implants and surgical tools. The exceptional biocompatibility, extremely regular porous structure, capacity for the formation of complex geometrical shapes and mechanical strength are the main qualities of these 3D bioceramics. 3Dbioceramics can also be produced cost-effectively and relatively quickly and, when incorporated into the human body, provide more safety and comfort for the patient and require less follow-up after surgery. Given these many attributes, the future of additive manufacturing of ceramics for biomedical applications is extremely bright and the market is expected to grow by leaps and bounds as healthcare systems seek to control spiralling patient care costs, and applications not previously considered advisable or possible continue to emerge.
The medical sector has always been interested in cutting-edge technologies, which is why 3DCeram began working with biomedical players in 2005. In subsequent years 3DCeram has focused on developing a mastery of the 3D printing ceramics process, 3D printers, ceramic materials and services encompassing maintenance and training. Today, the company is the undisputed global leader in the additive manufacturing of ceramics for biomedical applications.
3D Ceram leverages stereolithography (SLA) 3D printing technology to manufacture custom-made or small series bone substitutes and cranial or jawbone implants. The technology can be used to produce ceramic components layer by layer using a laser that polymerizes a paste composed of photosensitive resin and ceramic. The parts are then subjected to a heat treatment (debinding followed by sintering) that eliminates the resin and densifies the ceramic.
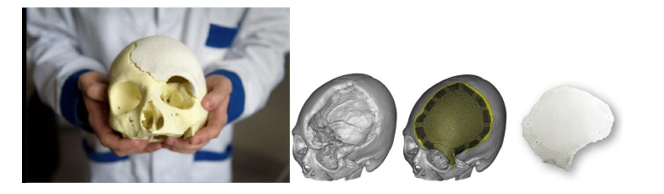
Custom-made HAP implant for the repair of large and complex craniofacial bone defects
With over a decade of medical 3D printing experience under its belt, 3DCeram produces a range of ceramic 3D printers and materials that are suitable for biomedical applications, including the accessible C100 EASY FAB system and the production-grade C3600 ULTIMATE. The company offers a number of materials that have been specifically formulated for biocompatibility and osteointegration, such as HAP (Hyd roxyapatite), TCP (Tricalcium Phosphate) and ATZ (Alumina Toughened Zirconia). 3DCeram’s products are suitable for many types of biomedical applications, from cranial and jawbone implants to dental devices.

Ceramaker 3600 ULTIMATE
In the biomedical sector, however, the ability to 3D print highly advanced or customized devices is not quite enough: all medical parts and products must undergo and meet stringent requirements. When it comes to the adoption of ceramic 3D printing in the medical sphere, certifications have not been a deal breaker, but they have created a bottleneck. CDCeram has pursued streamlining of the certification process for its biomedical customers through a new partnership with Gregory Nolens.
With a PhD in Biomedical Sciences and expertise in additive manufacturing and medical regulations, Gregory Nolens is uniquely equipped to help biomedical companies and players to not only implement ceramic 3D printing for medical device development and production, but to obtain the necessary certifications.
Contact
SRC is proud to represent 3DCeram in Canada and make available to our Canadian customers in the biomedical field the full range of 3DCeram products. Click here to contact SRC to learn more.