| Metallic alloys are frequently used under high thermal, chemical and mechanical stress. Numerous energy productions applications favor their oxidation, leading to a degradation of their properties. An extreme case is the nuclear power production, where zirconium based alloys are employed in nuclear reactors, at high temperature, under high humidity, and irradiation levels. The aeronautics industry is also concerned by the corrosion phenomena of alloys used in aircraft gas turbines that decrease their service life and/or their efficiency.
Thermogravimetric analysis (TGA) is a well-established experimental method to study the growth of oxide layers on metallic alloys and can be employed to study corrosion processes providing that the thermobalance used:
This is the case of several TGA designed and manufactured by SETARAM Instrument, more specifically SETSYS Evolution TGA and the symmetrical high temperature thermobalance TAG. |
||||
METHODSIsothermal A typical isothermal TGA data set is depicted in Figure 1, where the resistance to corrosion of an alloy plate was tested at 1100°C. The observed mass uptake (279,5 µg.cm-2) is due to the surface oxidation of the sample. After a fast mass increase, the diffusion of more oxygen through the already formed oxide layer becomes more and more difficult, leading to a deceleration of the oxidation reaction. Several other processes may have an effect on the TGA signal like breakaway, corresponding to mechanically driven rupture in the oxide layer and a subsequent fast increase of the oxidation rate.
Cyclic oxidationWhen testing the resistance to oxidation at high-temperature, the cyclic-oxidation may be preferred to replicate conditions close to the actual conditions of use. Indeed, it integrates isothermal oxidation kinetics, oxide-scale adherence, mechanical stresses, oxide creep and the evolution of these properties with time. Complex temperature profiles such as the one depicted by the red curve on Figure 2 can be easily programmed. The result is a stepwise mass increase due to the successive oxidation rate increases during heating and decreases during cooling. A chart showing the mass variation per step as a function of the cycle number can then be derived from the mass uptake signal.
Wet atmosphereIn most cases, humidity promotes corrosion, and experiments under dry conditions may not describe accurately the oxidation processes as they occur in wet atmospheres. This is why it is important to mention that SETARAM thermogravimetric analyzers can be connected to the WETSYS humid gas generator. WETSYS is designed for any application in which controlling the relative humidity rate of a chamber is necessary. It is a compact and automated wet gas generator based on a simple principle: the mixing of a dry gas and a water-saturated gas so as to maintain the relative humidity of the generated outlet gas at a given temperature. Both isothermal and cycling temperature profiles can be operated under wet conditions as the TGA temperature control is independent of the WETSYS.
APPLICATIONS
|
 Figure 5 – Setsys Evolution TGA Figure 5 – Setsys Evolution TGA |
Innovating approaches
At the Saint Etienne School of Mines, in the SPIN lab, an innovative setup involving the coupling between a TAG symmetrical thermogravimetric analyzer and acoustic emission was used to study the Zircaloy-4 corrosion mechanism [5].
The system, in which piezoelectric sensors were placed in the balance head and specific wave guides were employed to hold the samples in the TGA furnace, allowed simultaneous measurements of the sample mass variation and AE burst. Combined with post mortem characterization of oxidized samples, it provided additional information on the Zircaloy-4 corrosion mechanism:
- Kinetic transition is detected under air oxidation tests at 900°C by a change in the rate of mass gain and by the AE activity
- AE allowed distinguishing the cracks which occur during oxidation from the cracks linked with the cooling of the samples



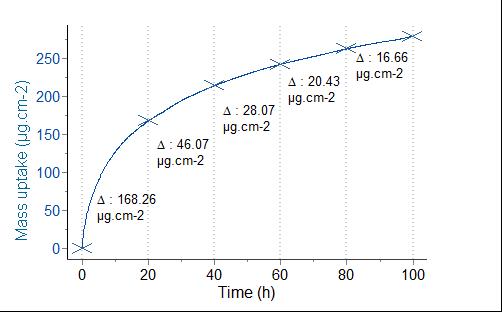
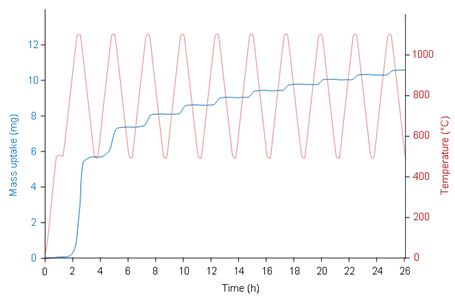
 Figure 3 –
Figure 3 –